🏀 📈 Upside Research: From Tissue to System: What Constitutes an Appropriate Response to Loading?
Title: From Tissue to System: What Constitutes an Appropriate Response to Loading?
Authors:
Tim J. Gabbett1
Eric Oetter 2
Tim J. Gabbett tim@gabbettperformance.com.au 1
Eric Oetter eoetter@grizzlies.com 2
Gabbett Performance Solutions, Brisbane, QLD 4011,
Memphis Grizzlies, Memphis, TN 38103, USA
Accepted: 20 September 2024
© The Author(s), under exclusive licence to Springer Nature Switzerland AG 2024
Initially Published online: 11 November 2024
Abstract
Optimal loading involves the prescription of an exercise stimulus that promotes positive tissue adaptation, restoring function in patients undergoing rehabilitation and improving performance in healthy athletes. Implicit in optimal loading is the need to monitor the response to load, but what constitutes a normal response to loading? And does it differ among tissues (e.g., muscle, tendon, bone, cartilage) and systems? In this paper, we discuss the “normal” tissue response to loading schema and demonstrate the complex interaction among training intensity, volume, and frequency, as well as the impact of these training variables on the recovery of specific tissues and systems.
Although the response to training stress follows a predictable time course, the recovery of individual tissues to training load (defined herein as the readiness to receive a similar training stimulus without deleterious local and/or systemic effects) varies markedly, with as little as 30 min (e.g., cartilage reformation after walking and running) or 72 h or longer (e.g., eccentric exercise-induced muscle damage) required between loading sessions of similar magnitude.
Hyperhydrated and reactive tendons that have undergone high stretch–shorten cycle activity benefit from a 48-h refractory period before receiving a similar training dose. In contrast, bone cells desensitize quickly to repetitive loading, with almost all mechanosensitivity lost after as few as 20 loading cycles.
To optimize loading, an additional dose (≤ 60 loading cycles) of bone-centric exercise (e.g., plyometrics) can be performed following a 4–8 h refractory period. Low-stress (i.e., predominantly aerobic) activity can be repeated following a short (≤ 24 h) refractory period, while greater recovery is needed (≥ 72 h) between repeated doses of high stress (i.e., predominantly anaerobic) activity. The response of specific tissues and systems to training load is complex; at any time, it is possible that practitioners may be optimally loading one tissue or system while suboptimally loading another.
The consideration of recovery timeframes of different tissues and systems allows practitioners to determine the “normal” response to load. Importantly, we encourage practitioners to interpret training within an athlete monitoring framework that considers external and internal load, athlete-reported responses, and objective markers, to contextualize load–response data.
1 Tissue Response to Load is Variable
As early as the 1940s, researchers demonstrated that the biochemical response of a tissue depended on the type of training that was performed [1]. In one of the few studies to document tissue-specific load injury, Orchard et al. [2] demonstrated that acute and chronic loads had differential effects on tendon, bone, joint, and muscles.
High chronic loads might be protective against some injuries (e.g., muscle injuries) but predictive of other injuries (e.g., joint injuries), while tendon injuries occur most frequently in response to large spikes and troughs in acute load.
High-intensity and maximal-effort running has been shown to alter tendon structure for 2 days [3, 4], with tendon structure returning to normal within 3–4 days. In healthy bone, mechanosensitivity is diminished after only 20 repetitive loading cycles [5], but is 90% restored within 8 h of rest; thus, further bone adaptation can be achieved if appropriate recovery time and an additional bone-centric stimulus is provided [6].
With such diverse tissue responses to an exercise stimulus, practitioners might wonder if “optimal” loading is even possible. If tissue responses to the same stimulus vary so greatly, is it possible that practitioners might be optimally loading one tissue, but suboptimally loading another? [7]
Picture: Gary Vitti, Kobe Bryant (LA Lakers / NBA)
In this narrative review, we discuss the “typical” tissue response to loading schema and demonstrate the complex interaction among training intensity, volume, and frequency. Training recommendations for specific tissues and systems are provided to assist practitioners in the development of rehabilitation and performance programs.
We searched the PubMed database, using combinations of the following key- words: (i) “training”, “load”, “exercise”, “intensity”, “vol- ume”, and “frequency”; (ii) “athlete” and “patient”; (iii) “tissue”, “muscle”, “tendon”, “bone” and “cartilage”; (iv) “response” and “recovery”. Individual systematic reviews and meta-analyses were hand-checked to identify any studies missed during the electronic database search, and only Eng- lish-language peer-reviewed publications involving human subjects were included.
2 Part 1: From Tissue to System—Different Loads Create Different Responses
Similar to tissue adaptation, the development of specific systems requires targeted exercise stimuli. Training load that lacks sufficient intensity will be inadequate to stress the anaerobic systems and engage fast-twitch muscle fibers [8–10], whereas frequent, higher volumes of intense train- ing may compromise recovery and/or aerobic adaptations [11].
It is well established that the development of sprint running performance is dependent on appropriate exposure to high-intensity sprinting, although sprint training activities can range from low (e.g., tempo runs at 60–70% maximal velocity) through high (e.g., resisted sprints, speed endurance, acceleration and maximal velocity at 80–98% maximal velocity) and supramaximal (e.g., assisted sprints, up to 105% maximal velocity) efforts [12].
Further complicating the load–response relationship for system development is that underlying physical qualities (e.g., strength and power) are often targeted to enhance sport-specific qualities (e.g.,speed), and the time course for recovery is dependent on the volume and intensity of training, joint range of motion, and contraction speed and type.
A minimum of 48 h recovery is recommended between consecutive resistance sessions utilizing the same muscle groups; longer recovery is required following sessions involving maximal intensity lifting owing to the large involvement of the central nervous system. Due to the high intensity of maximal-effort sprinting activities and the associated metabolic and tissue stress, best-practice recommendations involve 48–72 h recovery between high- intensity sprinting exposures [12, 13].
2.1 Physiological Responses to Exercise and Training
2.1.1 Acute and Chronic Adaptations to Training Stress
The acute and chronic adaptations to training stress depend on the type, volume, and intensity of exercise performed [14]. Neuromuscular adaptations predominantly occur in response to strength and power training, while endurance training involves both neuromuscular and cardiovascular adaptations. Classic endurance training results in enhanced stroke volume and cardiac output, maximal oxygen uptake (V̇ O2max), and mitochondrial biogenesis [15].
In contrast, strength training results in increased muscle cross-sectional area, enhanced motor unit recruitment and synchronization, and improved maximal force production [15]. Although exercise is commonly separated into long-duration, low- intensity exercise (involving the generation of low forces, predominantly aerobic energy release, and low central nervous system activation) and short-duration, high-intensity exercise (involving the generation of high forces, predominantly anaerobic energy release, and high central nervous system activation), energy systems function in an integrated manner, with very few activities deriving energy from any single system [16].
Equally, high-intensity training (in the form of strength training, high-intensity intermittent training, and sprint-interval training) has been shown to improve endurance performance [17, 18], while low-load strength training that approaches failure may lead to strength adaptations [19].
2.1.1.1 Endurance Training
Endurance training results in cardiovascular and musculoskeletal adaptations that support performance during prolonged aerobic exercise. The increased mitochondrial biogenesis and capillary density that occur with endurance training result in improved use of oxygen to generate energy [20]. These adaptations, along with changes in maximal cardiac output (driven predominantly by a higher stroke volume) [21], result in greater oxygen delivery to the working muscles and increases in exer- cise capacity and performance [22].
Although prolonged low-intensity exercise has predominantly been used to elicit aerobic adaptations, more recently, sprint-interval training (~ 30 s maximal bouts) and high- intensity interval training (~ 1–4 min near-maximal bouts) have been employed as a time-efficient way of improving endurance [15, 23]. High-intensity interval training has been shown to improve exercise capacity through adaptations in mitochondrial content and respiration after only 2 weeks of training [24].
Furthermore, skeletal muscle oxidative capacity and mitochondrial adaptations have been reported to be similar [25] or, in some cases, greater [26] following sprint- interval training than prolonged low-intensity exercise and high-intensity interval training. Recent evidence suggests that high-intensity training (i.e., sprint-interval training and high-intensity interval training) is mostly responsible for mitochondrial respiration and function adaptations, while prolonged low-intensity exercise increases skeletal muscle mitochondrial content [15].
Increases in creatine kinase, reductions in countermovement jump performance, and delayed-onset muscle soreness occur in the 24 h following high-intensity interval exercise [27]. Several researchers have investigated the effect of the high-intensity interval protocol on fatigue responses. Farias Junior et al. [28] assessed pressure-pain threshold, pressure- pain tolerance, and perceived pain intensity in the rectus femoris, biceps femoris, and gastrocnemius before and 24 h after either continuous exercise (20 min at 60% of maximal aerobic speed) or low-volume high-intensity interval exer- cise (10 × 60 s at 90% of maximal aerobic speed interspersed with 60 s of active recovery), in untrained men.
Both continuous and high-intensity interval exercise elicited delayed- onset muscle soreness, but no differences were observed between conditions. Wifison Alves et al. [29] compared the fatigue response with two low-volume high-intensity inter- val sessions (10 × 60 s versus 20 × 30 s, both performed at 100% of maximal aerobic speed).
No differences were found between conditions for muscle soreness and pain in the 24–48 h post-exercise. Wiewelhove et al. [27] reported greater fatigue (greater creatine kinase and delayed-onset muscle soreness, and larger reductions in countermovement jump height) in the 24 h following exercise in team sport athletes undergoing a sprint-interval protocol (4 × 6 × 5 s maximal-effort sprints) than following high-intensity inter- vals (ranging from 80 to 95% of velocity achieved in the 30–15 Intermittent Fitness Test). Borges et al. [30] com- pared the recovery profiles of young (mean age 25.9 years) and Masters (mean age 55.6 years) cyclists with a single high-intensity exercise session (6 × 30 s efforts at 175% peak power output with 4.5 min rest between efforts).
No significant group × time interactions were observed for maximal voluntary contraction, 10 s sprint, 30 min time trial performance, or creatine kinase, although perceptual recovery was reduced in the Masters athletes, with lower motivation and greater fatigue and muscle soreness in the 48 h post- exercise. Finally, Nuuttila et al. [31] compared physiological, perceptual, and performance responses to a 2-week block of either high-intensity interval training (ten sessions) or low-intensity training in recreational runners. Both training interventions elicited similar improvements in 3000-m running performance, although the high-intensity interval train- ing was associated with greater muscle soreness, reduced parasympathetic activity, and elevated sympathetic activity.
Collectively, these findings suggest that (1) the fatigue response to high-intensity interval training is comparable to that to continuous exercise, as long as total session volume is low, (2) maximal effort intervals (i.e., sprint intervals) are associated with longer recovery timeframes than high intensity intervals performed at lower intensities, (3) perceptual recovery may be prolonged in older athletes, and (4) while regular high-intensity interval training can be tolerated in the short term, adequate recovery is required between high- intensity interval sessions to optimize adaptations.
2.1.1.2 Strength and Power Training
In the early stages of a strength training program, improvements in strength are predominantly elicited through neural adaptations [32]. These adaptations include enhanced coordination, motor unit recruitment and synchronization [15].
Early unilateral limb strength training studies demonstrated the role of the central nervous system in strength adaptations [33];~8% improvements in strength were observed in the untrained contralateral limb, despite unchanged cross-sectional area [34]. This cross-transfer of strength, which is thought to be driven in part by localized muscle adaptations, cross-limb cortical interaction, and adaptations in spinal cord excit- ability [34] is greater in eccentric (~47%) than concentric (~ 28%) training [35].
Picture: Steph Curry (Golden State Warriors / NBA)
Morphological changes contribute more to longer-term improvements in strength [15, 36]. The force-generating capacity of a single muscle fiber is directly proportional to its cross-sectional area [36, 37]. Architectural factors such as fascicle length and pennation angle also influence force- generating capacity; longer fascicles allow greater force to be produced, optimizing the length–tension relationship [38]. Employing a range of motion that emphasizes training at long muscle lengths, and a repetition range between 2 and 8 s, is recommended for muscle hypertrophy adapta- tions [39].
In a recent systematic review and meta-analysis, Schoenfeld et al. [19] examined the effect of low-load (≤60% one-repetition maximum, 1RM) and high-load (> 60% 1RM) training on strength and hypertrophy adaptations. Greater improvement in 1RM (effect size 1.69 versus 1.32) was observed with high loads, while changes in isometric strength and muscle mass were comparable between high and low loads. These findings challenge the notion that an optimum repetition range exists for adaptations in lean muscle mass [40], and that both hypertrophy and isometric strength can occur across a range of loads and repetition ranges.
2.1.2 Muscle Adaptations
Eccentric strength training is commonly used to elicit muscle architectural adaptations in athletes that are thought to be beneficial for both performance and injury prevention [41].
For example, 12 sessions of eccentric hamstring training (using the Nordic hamstring exercise) resulted in increased fascicle length of the biceps femoris long head, along with improvements in increased concentric and eccentric knee flexion peak torque at 60°/s, concentric peak torque at 180°/s, and increased isometric knee flexion peak torque [41]. The effects of different strength and power training interventions (e.g., isometric, isolated eccentric, or sprinting) on muscle architecture and performance have been studied, although results are equivocal.
Sancese et al. [42] reported improvements in isokinetic strength with both Nordic hamstring exercise and sprint training performed across eight sessions, although no changes in sprint performance or mechanics were observed in either group. Timmins et al. [43] reported increases in biceps femoris long head fascicle length, thickness, and eccentric strength in response to either isometric or eccentric (Nordic hamstring exercise) training across a 38-week football season in semi-professional ath- letes. Improvements in acceleration and maximum velocity qualities occurred in the eccentric training group but not in the isometric training group [43].
Freeman et al. [44] reported improvements in eccentric hamstring strength in athletes who underwent either Nordic hamstring exercise or sprint training, but improvements in sprint performance were greater in the sprint training group. Equally, 6 weeks of sprint training was associated with greater increases in biceps femoris long head fascicle length, sprint performance, and mechanics than isolated hamstring training using the Nordic hamstring exercise [45].
Although there is conflicting evidence, these results demonstrate the muscle architectural adaptations that occur through both slow (e.g., Nordic ham- string exercise) and rapid (e.g., sprinting) eccentric activity; these changes are beneficial for mitigating hamstring injury risk and improving performance.
Lum and Howatson [46] studied the acute effects of a single session of either isometric strength training (back squat at four different knee angles, and Romanian deadlift and split squat at three different knee angles) or heavy resistance training (back squat at 85% 1RM, split squat at 40% 1RM squat, and Romanian deadlift at 95% 1RM squat) on subsequent 20-m sprint, countermovement jump, and isometric mid-thigh pull performance.
Performance was reduced in both groups immediately following training. However, while 20-m sprint, countermovement jump, and isometric mid- thigh pull performance returned to baseline values within 24 h in the subjects who performed isometric exercise, performance was still depressed following 24 h of recovery fol- lowing heavy resistance training. Following an acute bout, eccentric exercise is associated with greater strength loss [47], muscle damage, and soreness [48] than concentric-only exercise.
Although the muscle soreness following eccentric and isometric exercise is comparable, the reductions in strength persist for longer following eccentric exercise [47]. Following severe bouts of eccentric exercise, muscle damage biomarkers (e.g., creatine kinase, myoglobin) are elevated for up to 8 days [49], while (depending on the protocol) athlete-reported perceptions of soreness can remain elevated above baseline for 3 days [50, 51]. Responses to eccentric exercise follow a dose–response relationship, with low volumes associated with less pain [52], less muscle dam- age [49], and poorer strength adaptations [53] than higher volumes.
Rapid eccentric activity, typical of high-volume sprinting sessions, is associated with reductions in sprint performance and increases in hamstring strain injury risk factors [50, 54, 55]. Carmona et al. [54] showed that an acute, high-volume sprint dose (10 × 40-m maximal efforts, per- formed every 3 min) induced decrements in subsequent maximum sprinting performance (acceleration, maximum velocity, and horizontal and vertical force) that persisted for 48–72 h.
Reductions in posterior chain muscle strength, and lumbo-pelvic control, coupled with increases in muscle soreness and creatine kinase also occurred across this time period [54, 55]. Others have shown that residual fatigue (i.e., increased muscle damage and delayed-onset muscle soreness and reduced well-being, hamstring force production capac- ity, and physical performance) persists for > 72 h following team sport competition [56–59].
2.1.3 Tendon Adaptations
Tendons are sensitive to their mechanical environment [60]. Mechanical loading of tendon tissue results in upregulation of collagen expression and increased synthesis of collagen protein; the extent of protein synthesis is likely a function of the strain experienced by tenocytes [61, 62]. Collagen synthesis peaks ~ 24 h after exercise. Collagen degradation also occurs post-exercise, but peaks earlier than collagen synthesis [61].
Following a period of mechanical loading, tendon stiffness increases owing to changes in both tendon material and morphological properties [60, 63]. These changes in tendon properties help the storage and return of strain energy during locomotor (e.g., walking) and athletic (e.g., sprinting, jumping) tasks. A wide range of training interventions have been employed to increase the mechanical load on tendons, including varying training intensity, sets, repetitions, train- ing frequency per week, duration of a single loading cycle, and duration of the training intervention [60]. Of these, con- traction intensity and the duration of the training interven- tion appear to be most important.
In their 2015 systematic review and meta-analysis, Bohm et al. [60] showed that the effect sizes for changes in tendon stiffness were significantly greater (effect size 0.90 versus 0.04) when training with higher muscle contraction intensities (> 70% of maximal voluntary contraction or 1RM) than with lower contraction intensities. Longer intervention periods (≥ 12 weeks) are considered more effective at eliciting tendon adaptations than shorter interventions, although there is evidence that tendons respond positively to mechanical loading within 2 months [60]. The type of muscle contraction (i.e., iso- metric, concentric–eccentric, or eccentric only) has minimal effect on tendon adaptations [60].
It should be noted that, although contraction intensity and intervention duration appear to be the most important factors in changing the mechanical, material, and morphological properties of tendons, loading duration [64], frequency [65] and rate [64], joint angle [66], and repetitive versus static loading [64] are also important loading conditions that have been shown to influence tendon adaptations.
Healthy tendons respond well to consistent (i.e., daily) loading as long as stretch–shorten cycle activity and tendon strain are minimized [67]. Net loss of collagen may occur if less than 24 h recovery is provided between exercise bouts, leaving the tendon vulnerable to injury [61]. For patients with tendinopathy, less frequent loading is recommended [68]. For example, hyperhydrated and reactive tendons that have undergone high stretch–shorten cycle activity benefit from a 48-h refractory period before receiving a similar training dose. Heavy loads, including the use of isometric exercise, have been shown to induce beneficial changes in tendon architecture, while also reducing pain in patients with tendinopathy [68–71].
2.1.4 Bone Adaptations
Microdamage that occurs through repetitive loading is a necessary and normal component of bone adaptation. This microdamage results in bone-resorbing osteoclasts removing damaged regions of bone and osteoblasts forming new, undamaged bone [7].
In cortical bone, it may take up to 4 weeks for osteoclast activation and resorption to occur, while full mineralization of new bone may take between 3 and 12 months. This process is longer in trabecular bone [7].
Skeletal microdamage is dependent on both the number of times a bone is loaded and the magnitude (i.e., intensity) and the rate at which the load is applied [7]. Bone fatigue is most influenced by load magnitude, with a strong relationship reported between the magnitude of tissue stress/ strain and bone fatigue. Small increases in stress and strain result in large decreases in the number of bone cycles that can be performed before failure [7]. Weaker bones have lower stress and strain tolerance. Enhancing tolerance to bone stress requires the introduction of mechanical stress to promote skeletal adaptation and ensuring that the microdamage that occurs as a result of cumulative loading does not outweigh the ability of the bone to repair itself.
The adaptations that occur in bone differ between endurance and strength training. For example, endurance runners who participated only in their primary sport had lower bone density than those who also engaged in heavy strength train- ing [72].
Equally, retrospective studies have shown that runners [73] and military cadets [74] who did not participate in regular strength training were at increased risk of bone stress injury than habitually strength-trained participants. Multidirectional jumping, landing, and change-of-direction activities are recommended to build a robust skeleton [7]. Conversely, the unidirectional nature of running is not effective for building bone strength [7]. Bone cells desensitize to repetitive loading, with 95% of mechanosensitivity lost after as few as 20 loading cycles [5]. These findings have important implications for practitioners as they suggest that, after this mechanosensitivity is lost, further bone loading does not produce proportional increases in bone adaptation.
However, a 4–8 h refractory period restores mechanosensitivity and allows for a second small dose (≤ 60 loading cycles) of bone-centric exercise (e.g., plyometrics), potentially maximizing osteogenesis and adaptive potential in a given timeframe [7]. Although rehabilitation can be accelerated for some specific tissue injuries (e.g., muscle strains) [75], rehabilitation from bone stress injuries requires practitioners to respect a cautious and gradual recovery timeframe. For example, athletes with bone stress injuries are advised to be pain-free during daily activities for 5 consecutive days before commencing return-to-run programs [76].
2.1.5 Cartilage Adaptations
Although cartilage has the ability to deform and regain shape under the application and removal of forces [37], the effects of mechanical loading on cartilage regulation are unclear. Mechanical loading and cartilage thickness dose–response studies are limited, but it is generally recognized that adaptations of human cartilage to exercise are not linear [77].
Immobilization is associated with reduced cartilage thickness [78] while lower thigh muscle strength [79] and cross-sectional area [80] are associated with poorer cartilage morphology and more severe osteoarthritis after partial meniscectomy. In addition, in patients with osteoar- thritis, lower pain and disability symptoms were reported in those undergoing quadriceps-specific exercise than in those engaging in general lower limb exercises [81].
Cartilage has been shown to rapidly recover following mechanical loading [82–84]. Van Ginckel and Witvrouw [85] assessed the deformation of knee cartilage in response to squatting exercise in individuals with mild tibiofemoral osteoarthritis and healthy controls.
Reductions in cartilage volume occurred in both groups but returned to baseline within 15 min. No significant between-group differences were found for volume changes. Similar findings have been shown for talar cartilage volumes following squatting exercise; cartilage deformation occurred in response to loading but was restored to baseline within 30 min [86]. Beneficial adaptations that may occur in response to mechanical loading of cartilage tissue include stiffening of the pericellular and interterritorial matrix [87], increased cartilage volume and concentration of glycosa- minoglycans [88], and reduced cartilage loss [89]. Collec- tively, these findings suggest that strength training is a safe method of loading articular cartilage and plays a critical role in maintaining joint integrity and health.
Cartilage is an extremely resilient tissue that can with- stand large loads. Studies investigating the influence of exercise intensity on joint changes are equivocal, with some studies showing no effect [90] and others reporting greater deformation [91] with higher intensities.
Harkey et al. [90, 91] investigated deformation of the medial femoral cartilage in response to walking, running, and drop landing in healthy individuals. No differences in deformation were reported between walking (− 6.7%) and running (− 8.9%) [90], although cartilage deformation after the drop-landing task required longer time to recover compared with the walk- ing condition [91].
For example, ultra-endurance marathon runners experienced collagen disorientation and a decrease in the concentration of glycosaminoglycans in the femorotibial joint within the first 1100 km of the race, but no further changes in the subsequent 3500 km [92]. Equally, no new osteochondral lesions were found and no further softening of the cartilage occurred following completion of the race [92]. Although some studies have shown a greater preva- lence of hip and knee osteoarthritis in professional runners (13.3%) and sedentary individuals (10.2%) than in recrea- tional runners (3.5%), it was not possible to determine if these associations were causative or confounded by other risk factors, such as previous injury [93]. There is currently no strong evidence that strenuous exercise increases the risk of osteoarthritis in healthy joints [94–96].
3 Part 2: Programming Considerations for Practitioners
3.1 A Conceptual Framework for Determining the Appropriate Response to Loading
Given the wide range of tissue responses to a given load, how can practitioners provide practical, evidence-based training interventions to their patients and athletes? Based on the underpinning physiological responses and adaptations that occur during acute and chronic exercise, and the expected load–response, several recommendations for practitioners responsible for rehabilitation and performance training programs are provided below.
3.2 Prioritizing Tissue and System Loading for Healthy and Injured Athletes
We have recently presented a training model that describes the relative emphasis of local-tissue and sport-specific loading for healthy and injured athletes [97]. The loading emphasis for injured athletes is on restoring local-tissue capacity, while in healthy athletes, resources are predomi- nantly directed toward sport-specific loading.
However, an overemphasis on either approach can result in athletes with poor local tissue health (in athletes who overempha- size sport-specific loading) or poor sport-specific capacity (in athletes who overemphasize local-specific loading). It is widely suggested that successful rehabilitation and perfor- mance programs employ a combination of local-tissue and sport-specific loading [97, 98].
A challenge for practitioners is maintaining the function of healthy tissues in the presence of an injury to a specific tissue type. In injured athletes, practitioners should prioritize reloading of the vulnerable tissue while considering adaptive, supportive loading strategies for noncompromised tissues. When returning an athlete from bone stress injury, a more conservative loading approach may be required, even in the presence of healthy muscle or connective tissue [76]. However, there are evidence-based approaches that permit practitioners to “train around” the load-compromised bone. For example, the liberal use of blood flow restriction train- ing offers an ideal modality to maintain muscle adaptations [99] while minimizing joint and bone stress [100, 101]. The effects of blood flow restriction training on the mechanical properties of tendon (e.g., tendon stiffness) are equivocal, with some studies demonstrating positive connective tissue adaptations with low-load resistance training [102, 103] and others [104] showing no effect. In the presence of muscle injury, the health of connective tissue can be maintained through the use of short-to-long muscle length isometric exercise and eccentric training that employs progressive increases in force, speed, and time under tension. This pro- gression in eccentric activity incrementally prepares both the muscles and tendons for the higher-intensity stretch–shorten cycle activity demands (e.g., sprinting, jumping, changes of direction) required on return to competition.
3.3 If Unloading is Necessary, Maintain Global Load Capacity
Although load capacity is improved through graded exposure to training stress [105–107], there may be occasions where training loads need to be regressed before sensibly progress- ing [108]. In these instances, practitioners are encouraged to maintain global loading to minimize the systemic effects of detraining and moderate the risk of reinjury upon reintroducing sport-specific activities. The protective effect of high global capacity has recently been demonstrated in soc- cer players; players with higher chronic internal loads (as estimated using the session rating of perceived exertion) had reduced risk of injury at any given high-speed running volume compared with players with low chronic loads [109].
The protective effect of training has also been shown by other researchers. In men’s professional soccer, players were 87% more likely to be injured in the first match after return to play than the seasonal average injury rate. However, for each additional training session performed before returning to play, the risk of muscle injury was decreased by 13% [110]. Similar findings have been documented in Australian Rules football players; higher training loads (in terms of both more sessions and accumulation of greater high-speed running chronic loads) were associated with lower risk of reinjury on return to competition [111].
The majority of research has employed laboratory-based experimental designs using limb immobilization to study the effects of disuse. These studies have shown that healthy, inactive muscle tissue atrophies at ~ 0.5% per day [112, 113] with the greatest loss of muscle mass occurring in the initial 1–2 weeks of inactivity [114]. Systemic loading is commonly used during return-to-play protocols to comple- ment local tissue loading. Along with the maintenance of tissue architecture (e.g., collagen turnover in tendons and muscles) [62, 115] and neurophysiological performance determinants, the metabolic stress associated with systemic loading promotes adaptive hormonal secretions that can support tissue healing [116].
The benefits of systemic load- ing to prevent atrophy and strength losses associated with disuse can be found in studies encompassing a wide range of conditions and populations [117–119]. For example, in comparison with control patients, a combination of aerobic and strength training improved grip strength and walking speed in patients following hospitalization with myocardial infarction [118]. Similarly, a combination of aerobic and light resistance training resulted in significant increases in insulin-like growth factor-1 (IGF-1), walking speed, and strength in patients with multiple sclerosis compared with control subjects [117]. Case studies of elite soccer [120] and rugby [121] players have also shown the importance of maintaining systemic loading to prevent reductions in lean muscle mass, particularly during the early stages of injury.
This maintenance of strength and skeletal muscle [122] is driven in part through activation of the IGF-1 signaling pathway, which promotes myogenesis and inhibits protein degradation and cell death [119]. Furthermore, exercise has been associated with increases in brain-derived neurotrophic factor (BDNF), a neurotransmitter modulator that plays an important role in neuroplasticity, learning, and memory [123] as well as minimizing sleep and mood disturbances [124]. Given the importance of sleep to recovery processes [125, 126], and the need to reintegrate injured athletes into technical and tactical training post-injury [127], the increase in BDNF through exercise appears to play a critical role in the return-to-play process.
3.4 Consider Recovery Timeframes of Different Tissues and Systems When Determining the “Normal” Response to Load
Inter- and within-individual variability notwithstanding, the “typical” timeframes of recovery following training for different tissues and systems are relatively predictable and are shown as a conceptual framework in Fig. 1. After the initial training dose, performance is reduced, followed by a return to performance at or above (i.e., supercompensation) the initial levels [128, 129]. While it is well established that the intensity of load prolongs these recovery times, an understanding of the normal response to load allows practitioners to assess if the athlete has recovered from the training dose and is ready to receive a subsequent training dose.
For example, maximum-force isometric muscle contractions are associated with low fatigue and rapid recovery [46]. Compared with heavy strength training, maximum-force isometric muscle training results in smaller decrements in speed and isometric mid-thigh pull performance, and greater perceived recovery in the 24-h post-training window [46].
Given the postactivation performance-enhancing effects [130], and the rapid recovery following maximum-force isometric muscle contractions [46], this type of loading should be encouraged when training for sport-specific training adaptations. Conversely, sprinting elicits neuromuscular fatigue that is accompanied by a prolonged decrease in hamstring strength [55]. These reductions in hamstring strength are thought to be driven by both central and peripheral mechanisms (e.g., neuromuscular fatigue, muscle damage), which impair contractile function, but are restored within 48–72 h [12]. Importantly, for both muscles and tendons, low-intensity activity can be performed, and is even recommended, during
Fig. 1 Normal time course of recovery from loading bouts for different tissues and systems. The response of specific tissues and systems to training load is complex; at any time, it is possible that practitioners may be optimally loading one tissue or system while suboptimally loading another. Note that the response to exercise stress will be influenced by health and training status, with many health conditions associated with compromised tissue responses to training load.
The time course for adaptations of specific tissues and systems is based on the following references [4, 6, 7, 12, 27–29, 46–51, 54–58, 76, 90, 91]. CNS central nervous system the recovery window. Further evidence for the influence of intensity on recovery can be found from cartilage deformation [91], and tendon [60] and bone-loading [7] studies. Although cartilage recovery occurs rapidly (i.e., within 30 min) following walking and running, longer recovery periods (i.e., ≥ 45 min for cartilage thickness and cross- sectional area) may be required following higher-intensity drop-landing tasks [91]. Equally, even small increases in bone stress and strain significantly reduce the number of bone cycles that can be tolerated before failure [7], indicating the need for greater recovery following high-intensity bone loading.
3.5 ConsiderRecoveryTimeframesWhen Implementing High‐Intensity Training
Research has shown that high-intensity activity occurs at critical moments of competition (e.g., goals scored or con- ceded, shots on goal) [131–133]. These findings provide sup- port for the importance of high-intensity training for most sports. Equally, performance in individual sports requires athletes to maintain high speeds and power outputs over a prolonged period of time. The production and maintenance of these high-intensity efforts is extremely fatiguing, result- ing in both transient (60 min post-exercise) and longer-term (24–48 h) increases in muscle damage [56, 134–137], and reductions in well-being [56, 138–140] and neuromuscular function [56, 139].
Notwithstanding the wide range of periodization strategies employed by coaches [141], due to the fatiguing nature of high-intensity exercise, relatively small amounts of training time are devoted to these activities, even in well-designed programs. For example, in track sprinters, athletes typically only perform two high-intensity sprint ses- sions per week and keep high-intensity training volume to a minimum (e.g., 50–150 m of maximum velocity sprinting per session) [12].
Equally, despite the need to perform at high intensities during competition, endurance runners and cyclists (for events lasting approximately ≥ 2 min) devote most of their training time toward low-intensity training, with comparatively lower proportions (≤ 20%) performed at high intensities [11, 142]. This ensures (1) the allocation of sufficient training time to develop an aerobic capacity capable of supporting high-intensity activity, (2) the con- solidation of “high stress” training activities, (3) adequate recovery time between high-intensity sessions to promote training adaptations, and (4) that any high-intensity exercise performed is at an adequate intensity to prepare the tissues for the demanding nature of competition.
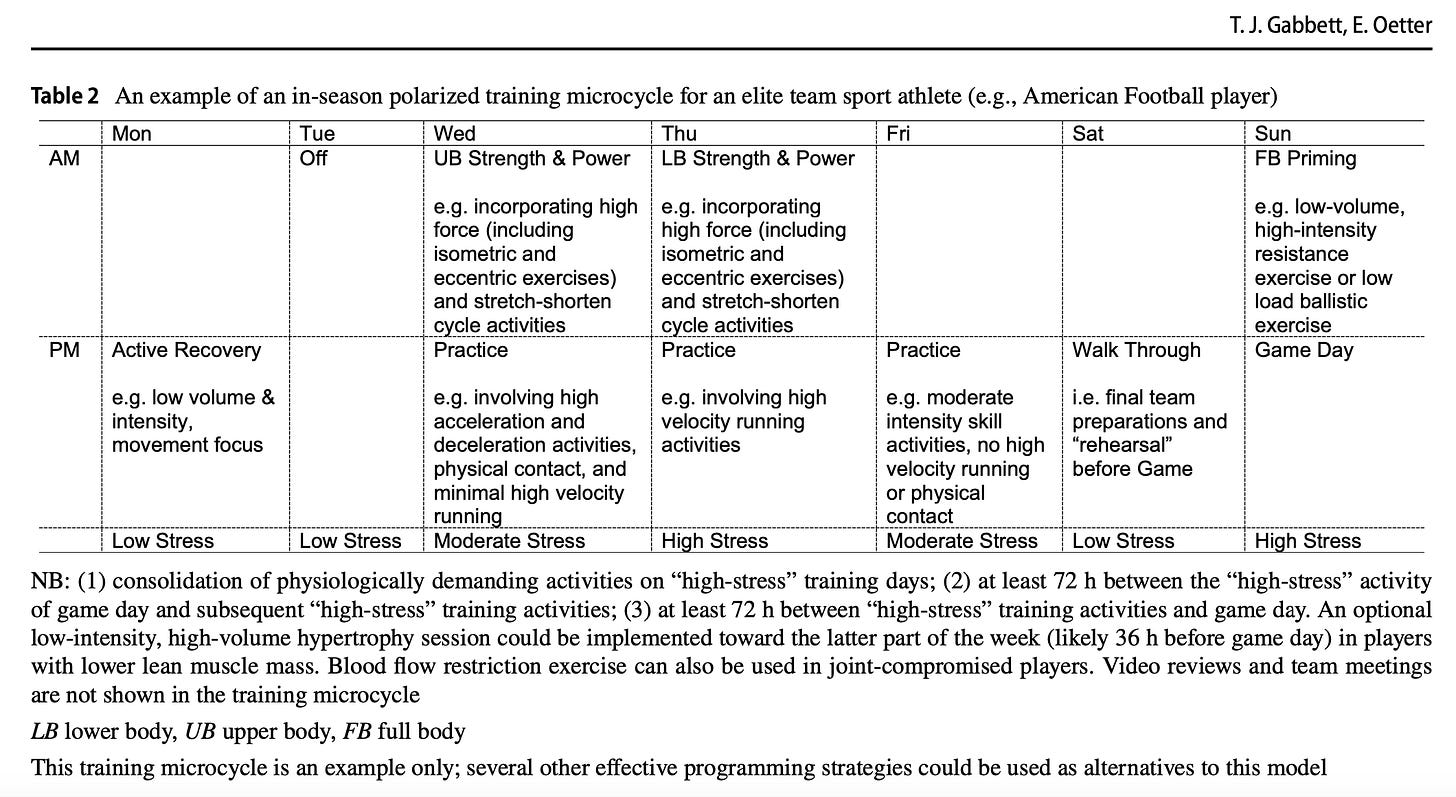
This polarized approach to training is not limited to individual or endurance sport athletes; team sports (such as American Football and elite soccer) will also use polarized training models to adequately prepare athletes for match play [143, 144], although it is acknowledged that micro-dosing may be the preferred programming strategy for sports with congested competition schedules [145].
Given that match day requires team sport athletes to perform highly demanding stretch–shorten cycle activities, practitioners must provide adequate recovery time between high-intensity training activities and those of com- petition. In this respect, the “lead-in” and “lead-out” time for match play are considered, with high-intensity training exposures typically occurring in the 48–72 h before and after competition [13, 146]. Examples of a polarized approach to training for individual sport (e.g., 10,000-m endurance run- ner) and team sport athletes (e.g., American Football) are provided in Tables 1 and 2, respectively.
Further evidence for the judicious use of high-intensity exercise within the training microcycle can be found in studies that have compared the inflammatory response to low-, moderate-, and high-intensity exercise [147, 148]. In a systematic review, Cerqueira et al. [147] found that the proinflammatory cytokine tumor necrosis factor alpha (TNF-α) only increased after high-intensity exercise, while interleukin-6 (IL-6) and interleukin-1β (IL-1β) increased more with high-intensity than with moderate-intensity exercise. Simi- lar findings have been found in patients undergoing regular physical activity; physically active patients with gout had fewer flares per year, decreased C-reactive protein levels, and less pain than patients who were physically inactive [149]. While high-intensity training is important for performance outcomes, collectively these findings suggest that adequate time is required between sessions to allow tissue recovery and training adaptations to occur.
Despite most practitioners recommending at least 48 h between high-intensity sessions or competition [146], there is some evidence from trained mountain bike cyclists that reducing the recovery period between high-intensity ses- sions has minimal effect on various performance measures. Hebisz et al. [150] compared 8 weeks of block training consisting of 17-day blocks of low-intensity training and 11-day blocks of sprint-interval and high-intensity interval train- ing with polarized training that simultaneously consisted of low- and high-intensity and sprint-interval training. While the polarized training program elicited greater improvements in V̇ O2max, no differences were observed between the block and polarized training programs for changes in maximal aerobic power, or the power achieved at the first and second ventilatory thresholds.
There is a large within- and inter-individual variation in response to loading [151, 152], with adaptation of most tis- sues (e.g., bone, tendon, and muscle) dependent on a range of factors, including loading conditions (e.g., intensity, dura- tion of single loading cycle, training frequency, repetitions, sets, duration of training intervention), exercise conditions (e.g., type of muscle contraction, joint angle, and whether the stimulus is applied statically or dynamically) [60], and even diurnal variation [153]. Of these factors, the intensity of loading is a significant determinant of the time course of recovery and degree of adaptation in tendons [60]. For example, tendon stiffness adaptations have been shown to be greater in exercise protocols employing high intensities (≥ 70% maximum voluntary contraction or 1RM) than in those using lower intensities [154, 155]. However, exces- sive mechanical loading can contribute to tendinopathy [62], which is associated with activity-related pain, focal tendon tenderness, and decreased strength and flexibility [156].
Both intensity and frequency of resistance training con- tribute positively to muscular strength, whereas resistance training volume contributes significantly to both muscular strength and hypertrophy [157]. Typically, 48 h of recovery is required between training sessions involving isotonic con- tractions with similar muscle groups; however, the intensity of training and contraction type will influence the timecourse of recovery [158]. Krentz and Farthing [158] demonstrated that intense eccentric exercise (six sets of eight maximal repetitions) performed every 2 days resulted in incomplete repair of muscle damage and prolonged impairment of mus- cle strength.
In a separate study, Bellosta-Lopez et al. [159] investigated the time course of recovery in pain perception, pressure pain sensitivity, active range of motion, maximal isometric strength, and muscle activity of the hamstrings during a maximal isometric contraction following a single- leg deadlift exercise (5 sets of 20 repetitions) designed to elicit delayed-onset muscle soreness. While muscle activity had recovered by day 4, pain perception, pressure pain sensitivity, maximal isometric strength, and active range of motion only returned to baseline on day 7. Given the heterogeneous time course of recovery to high-intensity muscle contractions, practitioners should consider both subjective and objective measures when determining an athlete’s readiness to perform subsequent training activities [160–163].
3.6 Interpreting Training Within an Athlete Monitoring Framework Allows Practitioners to Provide Context to Load–Response Data
Throughout this paper, we have highlighted that “load” and “response” data are more powerful when evaluated as a combined dataset; training loads can only be considered excessive or inadequate based on the response to that load. Equally, the athlete’s “response” (e.g., soreness, fatigue, mood, etc.) requires context of the desired training outcome; fatigue and soreness in preseason when training loads are high might be considered an appropriate response. However, a similar response might be considered undesirable leading into the first game of a seven-game finals series. We have recently proposed an athlete monitoring model that captures load–response data, allowing practitioners to evaluate the effectiveness of their training interventions and readiness of athletes to continue training [164, 165].
Although this model was designed with the “system” in mind, this athlete monitoring cycle, which encompasses external and internal load, subjective well-being, and objective measures of readiness, can also be applied to tissue stress (Fig. 2). This ath- lete monitoring framework describes a step-by-step strategy for interpreting data, from the exposure to a single training stimulus (or external load), through to the exposure of a sub- sequent training stimulus [164]. When combined with each previous step, practitioners can make informed decisions on training prescription (e.g., whether to adjust the external load during the session, identify if the athlete is tolerating the prescribed training load, and whether to introduce extra recovery or activation interventions to prepare the athlete for subsequent sessions) [164]. Below, we describe some of the assessment methods and technologies available to practitioners to monitor external and internal training load, and subjective and objective responses to that load.
3.6.1 External Load Monitoring
Predominantly employed by physical activity researchers, pedometers have been used extensively to count the number of steps an individual takes within a given time period (usually a day). Traditionally worn on the hip, these step counters are now integrated into smartphones and watches. Perhaps the most rudimentary of external load measures, research has shown strong associations between pedometers and accelerometers (r = 0.86) and measures of energy expenditure (r = 0.68), with accuracy better during walking and running speeds than during slow walking [166].
Global positioning systems are commonly used to measure external loads (e.g., total distance, high-speed running, sprinting, accelerations, and decelerations) in outdoor sports. However, given that this technology relies on satel- lites, quantifying the locomotor demands of indoor sports has predominantly been performed using labor-intensive video-based time–motion analysis [167]. Recently, local positioning systems (e.g., radio-frequency identification and ultra-wideband) have been used to quantify the external loads of athletes from indoor sports [168–172], with acceptable validity for most (e.g., position, distance, and average speed), but not all (e.g., instantaneous speed), measures of external load [168].
Global positioning systems also include inertial measurement sensors (e.g., triaxial accelerometers, gyroscopes, and magnetometers). This technology, which is worn on the body (and in other apparatus such as mouthguards and shoes), is now commonly used in a wide range of sports [173]. Accelerometers quantify accelerations on the body. The sum of the accelerations performed in three planes (and, therefore, an estimate of the external forces acting on the body) provides a measure of external biomechanical loads [173]. While inertial measurement systems worn on the upper back or sacrum can be used to provide an estimate of global external load, most practitioners working with running-, jumping-, and landing-based activities opt for a sensor worn on the shank or the insole of the shoe [174]. These sensors provide a closer approximation of ground reaction forces experienced in the lower body while also providing important information on gait deviations, ground contact times, and stride changes.
Presently, these devices provide the best available method of estimating external tissue loads when returning athletes from lower-limb complaints, such as bone stress injuries and Achilles tendon ruptures. An added advantage of the inertial sensors is that, when the obtained data are combined with tailored algorithms, there is potential to quantify sport-specific external loads [175].
In a systematic review of athlete-mounted inertial sensors, Chambers et al. [175] highlighted the use of this technology to detect sport-specific movements (e.g., throwing, tackling, fast bowling, swimming stroke classification, tennis serves, and snowboarding aerial maneuvers) from a wide range of individual, team, water, and snow sports. This is particularly important in sports where a large proportion of the external load arises from activities other than locomotion (i.e., high- speed running and sprinting).
3.6.2 Internal Load Monitoring
While the measurement of external loads is relatively straightforward, musculoskeletal modeling, which involves measurement of joint contact forces or muscle–tendon forces, is required to monitor the internal load of musculoskeletal tissues [173]. These direct measurements of internal tissue stress are difficult outside the laboratory environment, although surrogate measures can be used. For example, recent studies have proposed the use of wearable electromyography (EMG) technology to quantify internal loads and fatigue during exercise [176–179] and rehabilitation [180].
In comparison with laboratory-based EMG measures, wear- able EMG technology offers a valid method of monitoring a wide range of activities including orthostatic challenges [177], isometric exercise [177], treadmill running [181], and incremental cycle exercise to exhaustion [182]. This technology, which is located in a small (30 mm) patch worn on the skin surface or wearable shorts, is also sensitive to changes in exercise intensity [181, 182]. Others have used muscle oxygen saturation as a measure of internal load [183]. The rating of perceived exertion (RPE) scale is the most common method of monitoring global internal load of athletes. It is likely that some modification of the RPE scale or the use of differential RPE for leg muscle exertion is required to adequately capture internal local tissue loads [97].
3.6.3 SubjectiveWell‐BeingMeasures
Athlete-reported measures of soreness (e.g., muscle), mood disturbances (e.g., vigor, fatigue, stress, energy, recovery), or pain (e.g., bone and tendon) are by far the most common approach to assess tissue tolerance to training load [184]. These measures of subjective well-being are typically impaired with an acute increase in training load, with acute decreases in training load associated with improved subjective well-being [184]. Visual analog scales have been used to assess tendon pain [185]. Bone tissue damage can be estimated from excessive focal tenderness, swelling, pain at rest and on weight-bearing, and pain that increases with physical activity and fails to subside on cessation of physical activity [186].
3.6.4 Objective Measures of Physical Readiness
Finally, a myriad of different technologies can be used to provide objective data on the readiness of a tissue to receive a subsequent loading dose. For example, using isokinetic dynamometers, force plates, and velocity-based training systems, kinetic outputs can be used to probe the load tolerance and capacity of specific tissues during constrained isokinetic and/or isotonic actions (e.g., leg extension, counter- movement jump, bench throw). Shear wave elastography [187] and ultrasound tissue characterization [4] are methods to monitor tendon stiffness and morphology, respectively. Although dependent on access and likely impractical for daily use, these assays can be employed on a longer-term (e.g., 4-week) basis to gain feedback on intervention efficacy and monitor architectural changes that may be associated with tendinopathy. In the near future, it will be possible to use marker-less kinematic technologies to help impute specific loads incurred by tissue during sport movements [188, 189].
4 Advancement of Load–Response Research and Practice
4.1 Recommendations for Researchers
Although research has contributed to the understanding of recovery timeframes for specific tissues, there are sev- eral gaps that could be addressed to improve rehabilitation and training programs. Firstly, most [29, 30, 46, 48, 50, 54–56, 58, 135, 194], but not all [49, 51, 159], research has quantified the load–response over a short (i.e., usually 24–72 h) recovery period following loading, with at least some variables (e.g., sprint performance, biceps femoris long head muscle fiber structure, lumbopelvic control) not fully recovered 72 h after activities involving high-volume sprinting [54, 55], Therefore, it is not possible to state with certainty the exact duration of recovery required follow- ing specific loading for some tissues (e.g., rapid eccentric muscle contractions). Studying the response of specific tissues for longer post-loading windows is warranted.
Secondly, given that all training programs will have a pro- portion of “responders” and “stubborn responders,” [190] and that positive training adaptations can still be made with shorter recovery periods [145], identifying a “rap- idly adapting” athlete is of interest. If a subset of athletes can indeed be identified as “rapid adapters,” understand- ing the biopsychosocial characteristics that predict rapid adaptation would allow very specific training interventions to be introduced to develop this trait. Finally, although technological advancements have presented opportunities to measure a wide range of training loads and responses, many internal tissue loads cannot be directly measured outside the laboratory environment [173].
Furthermore, not all wearable devices are accompanied by software to process the raw signals (thereby requiring programming skills to process, filter, and interpret the acquired data), and the cost associated with some technologies makes the uptake of these devices beyond the reach of most clinics [179]. A challenge for researchers (and technology companies) is to develop valid and cost-effective athlete monitor- ing devices, capable of capturing the load–response, while also preserving the utility of the product.
4.2 Recommendations for Practitioners
Throughout this article, we have highlighted the importance of considering the tissue response when determining if an appropriate dose of training load has been applied. In this respect, monitoring either external or internal load in isolation will be inadequate to determine training adaptations. Indeed, the athlete monitoring framework described in Fig. 2 emphasizes the importance of using at least two sources of monitoring information to contextualize load–response data and inform subsequent training prescription. When presented with an injury, rehabilitation professionals are encouraged to first consider typical tissue recovery time- frames before initiating training programs.
Consideration of the local tissue capacity required to perform sporting skills is necessary to ensure appropriate local tissue loading is incorporated into the training schedule. Importantly, systemic loading is also recommended during rehabilitation to main- tain global capacity and prevent detraining of noninjured tissues. Solid clinical reasoning should always underpin the training process for both injured and healthy athletes, but the typical tissue and system load–response recommendations presented in Fig. 1 can be used as a starting point to guide training prescriptions.
5 Final Considerations
The response to exercise stress will be influenced by health and training status. Obesity increases the risk of tendinopathy, bone stress injury, and arthritic degeneration. Diabetes, rheumatoid arthritis, and hypercholesterolemia are considered risk factors for tendinopathy [191]. Low energy availability is associated with compromised skeletal muscle adaptation [192], low bone mineral density, and reduced cortical bone cross- sectional area [193]. While we have presented the “normal” response to loading for healthy tissues, these timelines will be influenced by advancing age, chronic medical conditions, energy balance, and other factors. In contrast, there is evidence to suggest that the time course of recovery may be shorter in individuals with better developed physical qualities [135, 194].
6 Conclusion
In summary, this paper describes the “normal” tissue response to training load and demonstrates the complex interaction among training intensity, volume, and frequency, as well as the impact of these training variables on the recovery of specific tissues and systems. The response of specific tissues and systems to training load is complex and is dependent on the magnitude of the training dose. The consideration of recovery timeframes of different tissues and systems allows practition- ers to determine the “normal” response to load. We encourage practitioners to interpret tissue and system stress within an athlete monitoring framework to contextualize load–response data.
Declarations
Funding No funding or grants from any public, commercial, or not- for-profit organizations were used in the preparation of this manuscript.
Conflict of Interest T.J.G. works as a consultant to several high-performance organizations, including sporting teams, artistic, industry, military, and higher-education institutions. E.O. has no potential conflicts of interest with the content of this article.
Data Availability No datasets were generated or analyzed for this narrative review.
Author Contributions T.J.G. conceptualized and wrote the first draft of the paper. E.O. provided critical feedback on subsequent drafts of the paper. Both authors take responsibility for the content of the paper. The authors would like to thank the reviewers for their constructive comments on the paper.
References
1. Viru A. Early contributions of Russian stress and exercise physi- ologists. J Appl Physiol. 2002;92:1378–82.
2. Orchard JW, Blanch P, Paoloni J, et al. Cricket fast bowling work- load patterns as risk factors for tendon, muscle, bone and joint injuries. Br J Sports Med. 2015;49:1064–8.
3. Docking SI, Daffy J, van Schie HTM, et al. Tendon structure changes after maximal exercise in the thoroughbred horse: use of ultrasound tissue characterisation to detect in vivo tendon response. Vet J. 2012;194(3):338–42. https://doi.org/10.1016/j. tvjl.2012.04.024.
4. Rosengarten SD, Cook JL, Bryant AL, et al. Australian foot- ball players’ Achilles tendons respond to game loads within 2 days: an ultrasound tissue characterisation (UTC) study. Br J Sports Med. 2015;49(3):183–7. https://doi.org/10.1136/bjspo rts-2013-092713.
5. Burr DB, Robling AG, Turner CH. Effects of biomechanical stress on bones in animals. Bone. 2002;30(5):781–.
Robling AG, Burr DB, Turner CH. Recovery periods restore mechanosensitivity to dynamically loaded bone. J Exp Biol. 2001;204:3389–99
Warden SJ, Edwards WB, Willy RW. Preventing bone stress injuries in runners with optimal workload. Curr Osteo- poros Rep. 2021;19(3):298–307. https://doi.org/10.1007/ s11914-021-00666-y.
Jansson E, Esbjörnsson M, Holm I, et al. Increase in the propor- tion of fast-twitch muscle fibres by sprint training in males. Acta Physiol Scand. 1990;140(3):359–63.
Dawson B, Fitzsimons M, Green S, et al. Changes in perfor- mance, muscle metabolites, enzymes and fibre types after short sprint training. Eur J Appl Physiol Occup Physiol. 1998;78(2):163–9.
Plotkin DL, Roberts MD, Haun CT, et al. Muscle fiber type transitions with exercise training: shifting perspectives. Sports. 2021;9:127. https://doi.org/10.3390/sports9090127.
Seiler S, Tonnessen E. Intervals, thresholds, and long slow dis- tance: the role of intensity and duration in endurance training. Sportsci. 2009;13:32–53.
Haugen T, Seiler S, Sandbakk O, et al. The training and develop- ment of elite sprint performance: an integration of scientific and best practice literature. Sports Med Open. 2019;5:44.
Cross R, Lovell R, Marshall PW, et al. Scheduling concurrent training 48 versus 72 h after simulated match play: effects on neuromuscular function and fatigue. Med Sci Sports Exerc. 2023;55(2):301–10.
Skorski S, Mujika I, Bosquet L, et al. The temporal relationship between exercise, recovery processes and changes in perfor- mance. Int J Sports Physiol Perform. 2019;14(8):1015–21.
Hughes DC, Ellefsen S, Baar K. Adaptations to endur- ance and strength training. Cold Spring Harb Perspect Med. 2018;8(6):a029769. https://doi.org/10.1101/cshperspect.a0297 69.
Gastin PB. Energy system interaction and relative contribution during maximal exercise. Sports Med. 2001;31:725–41.
Lum D, Barbosa TM, Aziz AR, Balasekaran G. Effects of isometric strength and plyometric training on running perfor- mance: a randomized controlled study. Res Q Exerc Sport. 2023;94:263–71.
Berryman N, Mujika I, Arvisais D, et al. Strength training for middle- and long-distance performance: a meta-analysis. Int J Sports Physiol Perform. 2018;13(1):57–63.
Schoenfeld BJ, Grgic J, Ogborn D, Krieger JW. Strength and hypertrophy adaptations between low- vs. high-load resistance training: a systematic review and meta-analysis. J Strength Cond Res. 2017;31:3508–23.
Coyle EF, Coggan AR, Hopper MK, Walters TJ. Determi- nants of endurance in well-trained cyclists. J Appl Physiol. 1985;64:2622–30.
Gledhill N, Cox D, Jamnik R. Endurance athletes’ stroke volume does not plateau: major advantage is diastolic function. Med Sci Sports Exerc. 1994;26:1116–21.
Bassett DR Jr, Howley ET. Limiting factors for maximum oxygen uptake and determinants of endurance performance. Med Sci Sports Exerc. 2000;32:70–84.
Buchheit M, Laursen PB. High-intensity interval training, solu- tions to the programming puzzle Part I: cardiopulmonary empha- sis. Sports Med. 2013;43:313–38.
Jacobs RA, Fluck D, Bonne TC, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and func- tion. J Appl Physiol. 2013;115:785–93.
Gibala MJ, McGee SL, Garnham AP, et al. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106:929–34.
Granata C, Oliveira RS, Little JP, et al. Training intensity modu-
lates changes in PGC-1α and p53 protein content and mitochon- drial respiration, but not markers of mitochondrial content in human skeletal muscle. FASEB J. 2016;30:959–70
Wiewelhove T, Fernandez-Fernandez J, Raeder C, et al. Acute responses and muscle damage in different high-intensity interval running protocols. J Sports Med Phys Fitness. 2016;56:606–15.
Farias-Junior LF, Browne RAV, Frazao DT, et al. Effect of low- volume high-intensity interval exercise and continuous exercise on delayed-onset muscle soreness in untrained healthy males. J Strength Cond Res. 2019;33:774–82.
Wifison Alves J, Farias-Junior LF, de Lucena Alves CP, et al. Low-volume high-intensity interval training sessions with differ- ent work-recovery durations and muscle damage in trained men. Res Q Exerc Sport. 2023;94:73–81.
Borges NR, Reaburn PR, Doering TM, et al. Age-related changes in physical and perceptual markers of recovery fol- lowing high-intensity interval cycle exercise. Exp Aging Res. 2018;44:338–49.
Nuuttila O, Nummela A, Kyrolainen H, et al. Physiological, perceptual, and performance responses to the 2-week block of high- versus low-intensity endurance training. Med Sci Sports Exerc. 2022;54:851–60.
Sale DG. Neural adaptation to resistance training. Med Sci Sports Exerc. 1988;20:S135–45.
Houston M, Froese E, Valeriote SP, et al. Muscle performance, morphology and metabolic capacity during strength training and detraining: a one leg model. Eur J Appl Physiol Occup Physiol. 1983;51:25–35.
Carroll TJ, Herbert RD, Munn J, et al. Contralateral effects of unilateral strength training: evidence and possible mechanisms. J Appl Physiol. 2006;101:1514–22.
Kidgell DJ, Frazer AK, Rantalainen I, et al. Increased cross- education of muscle strength and reduced corticospinal inhi- bition following eccentric strength training. Neuroscience. 2015;300:566–75.
Maestroni L, Read P, Bishop C, et al. The benefits of strength training on musculoskeletal system health: practical applications for interdisciplinary care. Sports Med. 2020;50:1431–50.
Ahtiainen JP. Physiological and molecular adaptations to strength training. In: Schumann M, Ronnestad BR. editors. Concurrent Aerobic and Strength Training. Springer International Publish- ing, AG. https://doi.org/10.1007/978-3-319-75547-2_5
Cormie P, McGuigan MR, Newton RU. Developing maximal neuromuscular power: part 1 – biological basis of maximal power production. Sports Med. 2011;41:17–38.
Androukalis Korakakis P, Wolf M, Coleman M, et al. Optimizing resistance training technique to maximize muscle hypertrophy: a narrative review. J Funct Morphol Kinesiol. 2023;9:9. https:// doi.org/10.3390/jfmk9010009.
Schoenfeld BJ, Grgic J, Van Every DW, Plotkin DL. Loading recommendations for muscle strength, hypertrophy, and local endurance: a re-examination of the repetition continuum. Sports. 2021;9(2):32. https://doi.org/10.3390/sports9020032.
Marusic J, Vatovec R, Markovic G, Sarabon N. Effects of eccen- tric training at long-muscle length on architectural and func- tional characteristics of the hamstrings. Scan J Med Sci Sports. 2020;30:2130–42.
Sancese A, Taylor L, Walsh G, et al. Effects of sprint versus strength training on risk factors for hamstring injury in football players. J Sports Med Phys Fitness. 2023;63:580–7.
Timmins RG, Filopoulos D, Giannakis J, et al. The effect of eccentric or isometric training on strength, architecture, and sprinting across an Australian football season. Med Sci Sports Exerc. 2024;56:564–74.
Freeman BW, Young WB, Talpey SW, et al. The effects of sprint training and the Nordic hamstring exercise on eccentric ham- string strength and sprint performance in adolescent athletes. J Sports Med Phys Fitness. 2019;59:1119–25.
Mendiguchia J, Conceicao F, Edouard P, et al. Sprint versus isolated eccentric training: comparative effects on hamstring architecture and performance in soccer players. PLoS ONE. 2020;15(2):e0228283. https://doi.org/10.1371/journal.pone. 0228283. (eCollection 2020).
Lum D, Howatson G. Comparing the acute effects of a session of isometric strength training with heavy resistance training on neuromuscular function. J Sci Sport Exerc. 2023. https://doi.org/ 10.1007/s42978-023-00241-0.
Royer N, Nosaka K, Doguet V, Jubeau M. Neuromuscular responses to isometric, concentric and eccentric contractions of the knee extensors at the same torque-time integral. Eur J Appl Physiol. 2022;122(1):127–39.
Clarkson PM, Byrnes WC, McCormick KM, et al. Muscle soreness and serum creatine kinase activity following iso- metric, eccentric, and concentric exercise. Int J Sports Med. 1986;7:152–5.
Rosvoglou A, Fatouros IG, Poulios A, et al. Recovery kinetics following eccentric exercise is volume-dependent. J Sports Sci. 2023;41:1326–3.
Cosio PL, Moreno-Simonet L, Porcelli A, et al. Assessment of inter-individual variability in hamstring muscle recovery after a sport-specific sprint training in women and men. Front Physiol. 2024;14:1331878. https://doi.org/10.3389/fphys.2023.1331878. (eCollection 2023).
Hasenoehrl T, Wessner B, Tschan H, et al. Eccentric resistance training intensity may affect the severity of exercise induced muscle damage. J Sports Med Phys Fitness. 2017;57:1195–204.
Behan FP, Opar DA, Vermeulen R, et al. The dose-response of pain throughout a Nordic hamstring exercise intervention. Scand J Med Sci Sports. 2023;33:542–6.
Behan FP, Vermeulen R, Whiteley R, et al. The dose-response of the Nordic hamstring exercise on biceps femoris architecture and eccentric knee flexor strength: a randomized interventional trial. Int J Sports Physiol Perform. 2022;17:646–54.
Carmona G, Moreno-Simonet L, Luis Cosio P, et al. Acute changes in hamstring injury risk factors following a session of high-volume maximal sprinting speed efforts in soccer players. Sports Health, 2024; (in press).
Carmona G, Moreno-Simonet L, Cosio PL, et al. Hamstrings on focus: are 72 hours sufficient for recovery after a football (soccer) match? A multidisciplinary approach based on hamstring injury risk factors and histology. J Sports Sci. 2024;42:1130–46.
Baumert P, Temple S, Stanley JM, et al. Neuromuscular fatigue and recovery after strenuous exercise depends on skeletal muscle size and stem cell characteristics. Sci Rep. 2021;11(1):7733. https://doi.org/10.1038/s41598-021-87195-x.
Silva JR, Rumpf MC, Hertzog M, et al. Acute and residual soccer match-related fatigue: a systematic review and meta- analysis. Sports Med. 2018;48:539–93.
Wiig H, Raastad T, Luteberget LS, et al. External load vari- ables affect recovery markers up to 72 h after semiprofessional football matches. Front Physiol. 2019;10:689. https://doi.org/ 10.3389/fphys.2019.00689. (eCollection 2019).
Gescheit DT, Cormack SJ, Reid M, Duffield R. Consecutive days of prolonged tennis match play: performance, physical, and perceptual responses in trained players. Int J Sports Phys- iol Perform. 2015;10(7):913–20.
Bohm S, Mersmann F, Arampatzis A. Human tendon adapta- tion in response to mechanical loading: a systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med Open. 2015;1(1):7. https://doi.org/10.1186/s40798-015-0009-9.
Magnusson SP, Langberg H, Kjaer M. The pathogenesis of
tendinopathy: balancing the response to loading. Nat Rev Rhe-
matol. 2010;6:262–8.
Kjaer M. Role of extracellular matrix in adaptation of ten-
don and skeletal muscle to mechanical loading. Physiol Rev.
2023;84:649–98.
Magnusson SP, Kjaer M. The impact of loading, unload-
ing, ageing and injury on the human tendon. J Physiol.
2019;597(5):1283–98.
Bohm S, Mersmann F, Tettke M, et al. Human Achilles tendon
plasticity in response to cyclic strain: efect of rate and dura-
tion. J Exp Biol. 2014;217(Pt 22):4010–7.
Arampatzis A, Peper A, Bierbaum S, Albracht K. Plasticity of
human Achilles tendon mechanical and morphological proper-
ties in response to cyclic strain. J Biomech. 2010;43:3073–9.
Kubo K, Ohgo K, Takeishi R, et al. Efects of isometric train-
ing at diferent knee angles on the muscle-tendon complex
in vivo. Scand J Med Sci Sports. 2006;16:159–67.
Muaidi QI. Rehabilitation of patellar tendinopathy. J Muscu-
loskelet Neuronal Interact. 2020;20(4):535–40.
Vladimirovna Pavlova A, Shim JSC, Moss R, et al. Efect of
resistance exercise dose components for tendinopathy manage-
ment: a systematic review with meta-analysis. Br J Sports Med.
2023;57:1327–34.
Rio E, Kidgell D, Purdam C, et al. Isometric exercise induces
analgesia and reduces inhibition in patellar tendinopathy. Br J
Sports Med. 2015;49(19):1277–83.
Kongsgaard M, Kovanen V, Aagaard P, et al. Corticosteroid
injections, eccentric decline squat training and heavy slow
resistance training in patellar tendinopathy. Scand J Med Sci
Sports. 2009;19(6):790–802.
Kongsgaard M, Qvortrup K, Larsen J, et al. Fibril morphol-
ogy and tendon mechanical properties in patellar tendinopathy:
efects of heavy slow resistance training. Am J Sports Med.
2010;38(4):749–56.
Duplanty AA, Levitt DE, Hill DW, et al. Resistance training
is associated with higher bone mineral density among young
adult male distance runners independent of physiological fac-
tors. J Strength Cond Res. 2018;32:1594–600.
Nussbaum ED, Bjornaraa J, Gatt CJ Jr. Identifying factors that
contribute to adolescent bony stress injury in secondary school
athletes: a comparative analysis with a healthy athletic control
group. Sports Health. 2019;11:375–9.
Rauh MJ, Macera CA, Trone DW, et al. Epidemiology of stress
fracture and lower-extremity overuse injury in female recruits.
Med Sci Sports Exerc. 2006;38:1571–7.
Bayer ML, Magnusson SP, Kjaer M. Early versus delayed
rehabilitation after acute muscle injury. N Engl J Med.
2017;377(13):1300–1. https://doi.org/10.1056/NEJMc1708134.
Warden SJ, Edwards WB, Willy RW. Optimal load for managing
low-risk tibial and metatarsal bone stress injuries in runners: the
science behind the clinical reasoning. J Orthop Sports Phys Ther.
2021;51(7):322–30. https://doi.org/10.2519/jospt.2021.9982.
Eckstein F, Hudelmaier M, Putz R. The efects of exercise on
human articular cartilage. J Anat. 2006;208:491–512.
Vanwanseele B, Eckstein F, Knecht H, et al. Longitudinal analy-
sis of cartilage atrophy in the knees of patients with spinal cord
injury. Arthritis Rheum. 2003;48:2377–81.
Ericsson YB, Roos EM, Owman H, et al. Association between
thigh muscle strength four years after partial meniscectomy
and radiographic features of osteoarthritis 11 years later. BMC
Musculoskel Disord. 2019;20:512. https://doi.org/10.1186/
s12891-019-2875-7.
Hudelmaier M, Glaser C, Englmeier KH, et al. Correlation of
knee-joint cartilage morphology with muscle cross-sectional
areas vs. anthropometric variables. Anat Rec A Discov Mol Cell
Evol Biol. 2003;270:175–84.
Juhl C, Christensen R, Roos EM, et al. Impact of exercise type
and dose on pain and disability in knee osteoarthritis: a system-
atic review and meta-regression analysis on randomized con-
trolled trials. Arthritis Rhematol. 2014;66:622–36.
Cutclife HC, Davis KM, Spritzer CE, et al. The character-
istic recovery time as a novel, noninvasive metric for assess-
ing in vivo cartilage mechanical function. Ann Biomed
Eng. 2020;48(12):2901–10. https:// doi. org/ 10. 1007/
s10439-020-02558-1.
Coburn SL, Crossley KM, Kemp JL, et al. Is running good or bad
for your knees? A systematic review and meta-analysis of car-
tilage morphology and composition changes in the tibiofemoral
and patellofemoral joints. Osteoarth Cartil. 2023;31:144–57.
Khan MCM, O’Donovan J, Charlton JM, et al. The infuence of
running on lower limb cartilage: a systematic review and meta-
analysis. Sports Med. 2022;52:55–74.
Van Ginckel A, Witvrouw E. Acute cartilage loading responses
after an in vivo squatting exercise in people with doubtful to
mild knee osteoarthritis: a case-control study. Phys Ther.
2013;93(8):1049–60. https://doi.org/10.2522/ptj.20120491.
Van Ginckel A, Roosen P, Almqvist KF, et al. Effects of
in vivo exercise on ankle cartilage deformation and recovery
in healthy volunteers: an experimental study. Osteoarthr Cartil.
2011;19(9):1123–31. https://doi.org/10.1016/j.joca.2011.06.009.
Bricca A, Juhl CB, Grodzinsky AJ, et al. Impact of a daily
exercise dose on knee joint cartilage—a systematic review and
meta-analysis of randomized controlled trials in healthy animals.
Osteoarthr Cartil. 2017;25:1223–37.
Bricca A, Juhl CB, Steultjens M, et al. Impact of exercise on
articular cartilage in people at risk of, or with established, knee
osteoarthritis: a systematic review of randomised controlled tri-
als. Br J Sports Med. 2019;53:940–7.
Amin S, Baker K, Niu J, et al. Quadriceps strength and the risk
of cartilage loss and symptom progression in knee osteoarthritis.
Arthritis Rheum. 2009;60:189–98.
Harkey MS, Blackburn JT, Davis H, et al. Ultrasonographic
assessment of medial femoral cartilage deformation acutely
following walking and running. Osteoarthritis Cartilage.
2017;25(6):907–13. https://doi.org/10.1016/j.joca.2016.12.026.
Harkey MS, Blackburn JT, Hackney AC, et al. Comprehensively
assessing the acute femoral cartilage response and recovery after
walking and drop-landing: an ultrasonographic study. Ultrasound
Med Biol. 2018;44(2):311–20. https://doi.org/10.1016/j.ultra
smedbio.2017.10.009.
Schutz U, Ehrhardt M, God S, et al. A mobile MRI feld study
of the biochemical cartilage reaction of the knee joint during a
4,486 km transcontinental multistage ultra-marathon using T2*
mapping. Sci Rep. 2020;10(1):8157. https://doi.org/10.1038/
s41598-020-64994-2.
Alentorn-Geli E, Samuelsson K, Musahl V, et al. The associa-
tion of recreational and competitive running with hip and knee
osteoarthritis: a systematic review and meta-analysis. J Orthop
Sports Phys Ther. 2017;47:373–90.
Sandmeier RH. Osteoarthritis and exercise: does increased activ-
ity wear out joints? Perm J. 2000;4:26–8.
Burfeld M, Sayers M, Buhmann R. The association between
running volume and knee osteoarthritis prevalence: a system-
atic review and meta-analysis. Phys Ther Sport. 2023;61:1–10.
https://doi.org/10.1016/j.ptsp.2023.02.003.
Migliorini F, Marsilio E, Oliva F, et al. Elderly runners and
osteoarthritis: a systematic review. Sports Med Arthrosc Rev.
2022;30:92–6.
Gabbett T, Sancho I, Dingenen B, et al. When progressing train-
ing loads, what are the considerations for healthy and injured
athletes? Br J Sports Med. 2021;55(17):947–8. https://doi.org/
10.1136/bjsports-2020-103769.
Buckthorpe M, Della Villa F, Della Villa S, Roi GS. On-feld
rehabilitation part 2: a 5-stage program for the soccer player
focused on linear movements, multidirectional movements,
soccer-specifc skills, soccer-specifc movements, and modifed
practice. J Orthop Sports Phys Ther. 2019;49:570–5.
Miller BC, Tirko AW, Shipe JM, et al. The systemic efects of
blood fow restriction training: a systematic review. Int J Sports
Phys Ther. 2021;16:978–90.
Bowman EN, Elshaar R, Milligan H, et al. Proximal, distal, and
contralateral efects of blood fow restriction training on the
lower extremities: a randomized controlled trial. Sports Health.
2019;11(2):149–56. https://doi.org/10.1177/1941738118821929.
De Renty C, Forelli F, Mazeas J, et al. Knee loading with blood
fow restriction can enhance recovery after total knee arthro-
plasty. Cureus. 2023;15(4):e37895. https://doi.org/10.7759/
cureus.37895.
Burton I, McCormack A. Blood fow restriction resistance train-
ing in tendon rehabilitation: a scoping review on intervention
parameters, physiological efects, and outcomes. Front Sports
Act Liv. 2022. https://doi.org/10.3389/fspor.2022.879860.
Centner C, Lauber B, Seynnes OR, et al. Low-load blood fow
restriction training induces similar morphological and mechani-
cal Achilles tendon adaptations compared with high-load resist-
ance training. J Appl Physiol. 2019;127:1660–7.
Kubo K, Komuro T, Ishiguro N, et al. Efects of low-load resist-
ance training with vascular occlusion on the mechanical proper-
ties of muscle and tendon. J Appl Biomech. 2006;22:112–9.
Dye SF. The pathophysiology of patellofemoral pain: a tissue
homeostasis perspective. Clin Ortho Rel Res. 2005;436:100–110.
Gabbett TJ. The training—injury prevention paradox: should
athletes be training smarter and harder? Br J Sports Med.
2016;50:273–80.
Verhagen E, Gabbett T. Load, capacity and health: critical
pieces of the holistic performance puzzle. Br J Sports Med.
2019;53(1):5–6. https://doi.org/10.1136/bjsports-2018-099819.
(Epub 2018 Sep 25).
Gabbett TJ. The training-performance puzzle: how can the past
inform future training directions? J Athl Train. 2020;55(9):874–
84. https://doi.org/10.4085/1062/6050.422.19.
Malone S, Owen A, Mendes B, et al. High-speed running
and sprinting as an injury risk factor in soccer: can well-
developed physical qualities reduce the risk? J Sci Med Sport.
2018;21(3):257–62. https://doi.org/10.1016/j.jsams.2017.05.016.
Bengtsson H, Ekstrand J, Walden M, et al. Few training sessions
between return to play and frst match appearance are associ-
ated with an increased propensity for injury: a prospective cohort
study of male professional football players during 16 consecutive
seasons. Br J Sports Med. 2020;54(7):427–32. https://doi.org/10.
1136/bjsports-2019-100655.
Stares J, Dawson B, Peeling P, et al. How much is enough in reha-
bilitation? High running workloads following lower limb muscle
injury delay return to play but protect against subsequent injury. J
Sci Med Sport. 2018;21(10):1019–24. https://doi.org/10.1016/j.
jsams.2018.03.012.
Phillips S, Glover E, Rennie M. Alterations of protein turno-
ver underlying disuse atrophy in human skeletal muscle. J Appl
Physiol. 2009;107:645–54.
You may also like: